Redox Signaling in Plants
Francisco J. Cejudo and Juan M. Pérez-RuízThe central objective of our group is to establish the functional relationship between the different components of the complex regulatory machinery of plant chloroplast redox regulation, which allows the rapid acclimation of photosynthetic metabolism to environmental cues such as changes in light intensity and temperature. It is also a goal of our group to identify the function of these redox systems in chloroplast biogenesis, thus, in early stages of plant development, such as embryogenesis during seed development and cotyledon differentiation during post-germinative growth.
To address these objectives, we analyze the impact of redox regulation on photosynthetic efficiency, carbon metabolism, and lipid metabolism with emphasis on the mechanisms controlling the degree of fatty acid unsaturation. We also analyze the function of plastid redox systems in the control and mode of action of molecular chaperones and their impact on chloroplast biogenesis and stability. Furthermore, we try to obtain a global view of redox regulation in plants, both in adult leaves and at early stages of development, using a wide range of methodologies such as RNASeq-based transcriptomic analyses and global and redox proteomics.
Most of these goals are addressed using the model plant Arabidopsis thaliana by applying a combination of experimental approaches, which include: generation of mutant plants by gene editing via the CRISPR/Cas9 method, expression of transgenes, physiological analyses of photosynthetic parameters based on chlorophyll fluorescence, biochemical analyses of protein-protein interactions based on immunoprecipitation assays, alkylation assays to determine the in vivo redox state of chloroplast proteins, as well as enzymatic assays with purified enzymes. We also perform cell biology studies based on confocal microscopy, electronic transmission microscopy, and lipid analyses by chromatographic techniques.
An additional objective of our group is to explore the biotechnological applications of the chloroplast redox systems to improve plant tolerance to temperature stress. This objective is addressed in the crop plant Camelina sativa, an oilseed plant of agronomic interest whose yield is highly compromised by heat stress.
Present financial support
Ref.: PID2020-115156G. Desentrañando el componente oxidativo de la regulación redox del cloroplasto. AEI, Ministerio de Ciencia e Innovación.
Ref.: EMSL Large-Scale Proposal 60307. The thiol redox proteome dynamics in Arabidopsis thaliana in response to light. Environmental Molecular Science Laboratory (EMSL), EE. UU
Ref.: CNS2023-144424. Coordinación de las distintas vías de transporte fotosintético de electrones como clave para la aclimatación de las plantas a distintas condiciones lumínicas / Interplay between different photosynthetic electron transport pathways as key for plant light acclimation. AEI, Ministerio de Ciencia e Innovación

- Pérez-Ruiz JM, Guinea M, Puerto-Galán L, Cejudo FJ. NADPH thioredoxin reductase C is involved in redox regulation of the Mg-chelatase I subunit in Arabidopsis thaliana chloroplasts. Mol Plant. 2014 Jul;7(7):1252-5. doi: 10.1093/mp/ssu032
- Naranjo B, Mignée C, Krieger-Lizskay A, Hornero-Méndez D, Gallardo-Guerrero L, Cejudo FJ, Lindahl M. The chloroplast thioredoxin reductase C, NTRC, controls non-photochemical quenching of light energy and photosynthetic electron transport in Arabidopsis. Plant Cell Environ. 2016 Apr;39(4):804-22. doi: 10.1111/pce.12652
- Ojeda V, Pérez-Ruiz JM, González M, Nájera VA, Sahrawy M, Serrato A, Geigenberger P, Cejudo FJ. NADPH thioredoxin reductase C and thioredoxins act concertedly in seedling development. Plant Physiol. 2017 Jul;174(3):1436-1448. doi: 10.1104/pp.17.00481
- Pérez-Ruiz JM, Naranjo B, Ojeda V, Guinea M, Cejudo FJ. The NTRC-dependent redox balance of 2-Cys peroxiredoxins is needed for optimal function of the photosynthetic apparatus. Proc Natl Acad Sci U S A. 2017 Nov 7;114(45):12069-12074. doi: 10.1073/pnas.1706003114
- Ojeda V, Pérez-Ruiz JM, Cejudo FJ. 2-Cys peroxiredoxins participate in the oxidation of chloroplast enzymes in the dark. Mol Plant. 2018 Nov 5;11(11):1377-1388. doi: 10.1016/j.molp.2018.09.005
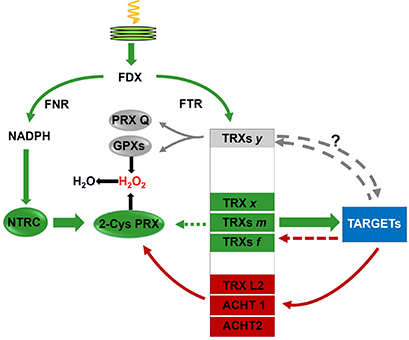
- Cejudo FJ, González MC, Pérez-Ruiz JM (2021) Redox regulation of chloroplast metabolism. Plant Physiol. 2021 May 27;186(1):9-21. doi: 10.1093/plphys/kiaa062
- Ojeda V, Jiménez-López J, Romero-Campero FJ, Cejudo FJ, Pérez-Ruiz JM. A chloroplast redox relay adapts plastid metabolism to light and affects cytosolic protein quality control. Plant Physiol. 2021 Sep 4;187(1):88-102. doi: 10.1093/plphys/kiab246
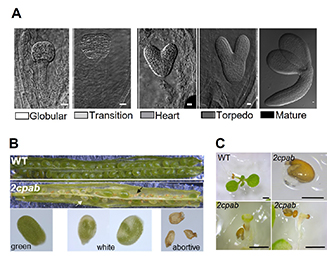
- Gallardo-Martínez AM, Jiménez-López J, Hernández ML, Pérez-Ruiz JM, Cejudo FJ. Plastid 2-Cys peroxiredoxins are essential for embryogenesis in Arabidopsis. Redox Biol. 2023 Jun;62:102645. doi: 10.1016/j.redox.2023.102645
- Casatejada A, Puerto-Galán L, Pérez-Ruiz JM, Cejudo FJ. The contribution of glutathione peroxidases to chloroplast redox homeostasis in Arabidopsis. Redox Biol. 2023 Jul;63:102731. doi: 10.1016/j.redox.2023.102731
- Hernández ML, Jiménez-López J, Cejudo FJ, Pérez-Ruiz JM. 2-Cys peroxiredoxins contribute to thylakoid lipid unsaturation by affecting FAD8 abundance in Arabidopsis chloroplasts. Plant Physiol. 2024 Feb 22:kiae102. doi: 10.1093/plphys/kiae102


