Mechanisms of adaptation of plants to abiotic stresses
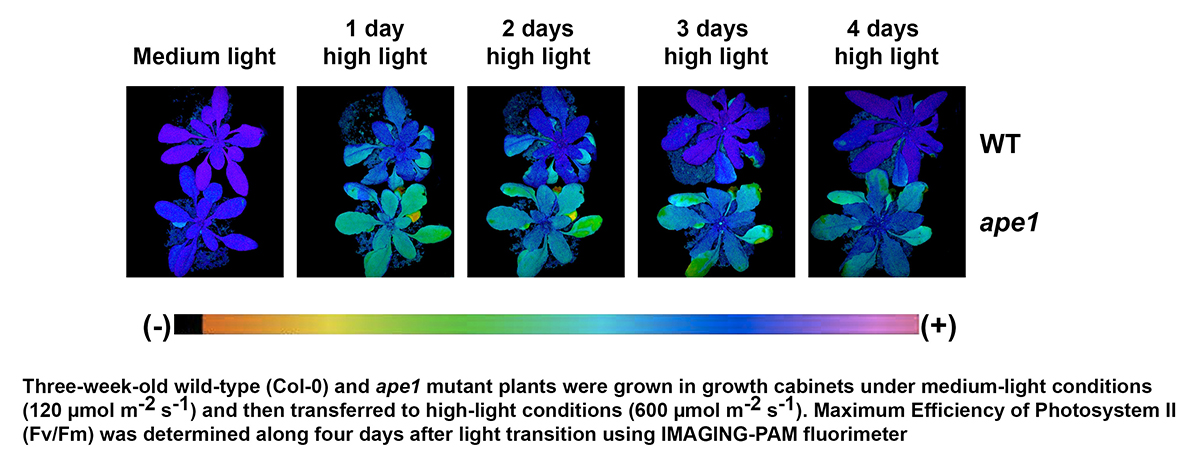
Ángel Mérida and M. Cruz GonzálezOur Group has extensive experience in studying carbon metabolism (Ángel Mérida) and different systems of redox regulation in plants (María-Cruz González). Most of these studies have been conducted in the chloroplast, an organelle essential for understanding much of plant metabolism, as well as their responses to different environmental stimuli. Continuing with the study of chloroplastic metabolism, we are currently analyzing how certain environmental stimuli affect the metabolism of this organelle. Specifically, we are studying how light intensity affects an essential process for plant development, such as photosynthesis, and the mechanisms existing in the chloroplast that allow the plant to acclimate to fluctuating light intensity situations. One of these adaptation mechanisms involves the action of the protein APE1 (Acclimation of Photosynthesis to Environment 1). This is a chloroplastic protein located in the thylakoid membranes and is found in all photosynthetic organisms, from cyanobacteria to higher plants, showing a high degree of conservation. Deleting this protein in Arabidopsis thaliana prevents the Maximum Efficiency of Photosystem II (Fv/Fm) from adapting to high light intensity, keeping this parameter value below what is detected in the wild-type plant. Although the APE1 protein was identified over twenty years ago, the molecular mechanisms of action of this protein are still unknown. Our Group is investigating how the elimination of APE1 alters various photosynthetic parameters depending on the light intensity received by the plant, as well as the molecular alterations that occur in the photosynthetic apparatus under those conditions. Similarly, we are interested in identifying the proteins that interact with APE1 to carry out its function. These studies are being conducted in the model plant Arabidopsis. However, as mentioned earlier, APE1 is found in all photosynthetic organisms, so one of our goals is to apply the results obtained in Arabidopsis to other economically important plants.

| Name | Surname | Category | Phones | |
|---|---|---|---|---|
| Francisco José | Arana Roncero | Researcher Contract | ext. 446059 | |
| Mari Cruz | González García | US Tenured Professor | 954489647 ext. 446047 |
|
| Ángel | Mérida Berlanga | CSIC Tenured Scientist | 954489507 ext. 446007 |
|
| Diego | Torres Romero | Postdoctoral Researcher | ext. 446059 |
- Torres-Romero D, Gómez-Zambrano A, Serrato AJ, Sahrawy M, Mérida A (2023) Arabidopsis fibrillin 1-2 subfamily exerts their functions via specific protein-protein interactions. Journal of Experimental Botany. 73, pp.903-914. https://doi.org/10.1093/jxb/erab452
- Delgado-Requerey V, Cejudo, FJ, Gonzalez, MC (2023) The functional relationship between NADPH thioredoxin reductase C, 2-Cys peroxiredoxins, and m-type thioredoxins in the regulation of Calvin-Benson cycle and malate-valve enzymes in Arabidopsis Antioxidants. 12. Issue 5. https://doi.org/10.3390/antiox12051041
- Serrato AJ, Rojas-González JA, Torres-Romero D, Vargas P, Mérida A, Sahrawy M. (2021) Thioredoxins m are major players in the multifaceted light-adaptive response in Arabidopsis thaliana. Plant Journal 108: 120-133. https://doi.org/10.1111/tpj.15429
- Mérida A, Fettke J (2021) Starch granule initiation in Arabidopsis thaliana chloroplasts. The Plant Journal. 107, pp.688-697. https://doi.org/10.1111/tpj.15359
- González MC, Cejudo FJ, Sahrawy M, Serrato AJ (2021) Current knowledge on mechanisms preventing photosynthesis redox imbalance in plants. Antioxidants Vol 10. Issue 11. https://doi.org/10.3390/antiox10111789
- González MC, Delgado-Requerey V, Ferrández J, Serna A, Cejudo FJ (2019) Insights into the function of NADPH thioredoxin reductase C (NTRC) based on identification of NTRC-interacting proteins in vivo. Journal of Experimental Botany 70: 5787-5798. https://doi.org/10.1093/jxb/erz326
- Ojeda V, Pérez-Ruiz JM, González MC, Nájera VA, Sahrawy M, Serrato AJ, Geigenberger P, Cejudo FJ (2017) NADPH thioredoxin reductase C and thioredoxins act concertedly in seedling development. Plant Physiology 174: 1436-1448. https://doi.org/10.1104/pp.17.00481
- Raynaud S, Ragel P, Rojas T, Mérida A (2016) The N-terminal part of Arabidopsis thaliana Starch Synthase 4 determines the localization and activity of the enzyme. Journal of Biological Chemistry. 291, pp.10759-10771. https://doi.org/10.1074/jbcM115.698332
- Gámez-Arjona FM, Raynaud S, Ragel P, Mérida A (2014) Starch synthase 4 is located in the thylakoid membrane and interacts with plastoglobule-associated proteins in Arabidopsis. The Plant Journal. 80, pp.305-316. https://doi.org/10.1111/tpj.12633
- Ragel P, Streb S, Feil R, Sahrawy M, Annunziata MG, Lunn JE, Zeeman S, Mérida A (2013) Loss of starch granule initiation has a deleterious effect on the growth of Arabidopsis plants due to an accumulation of ADP-Glucose. Plant Physiology. 163, pp.75-85. https://doi.org/10.1104/pp.113.223420


