Gene expression and metabolic regulation in cyanobacteria
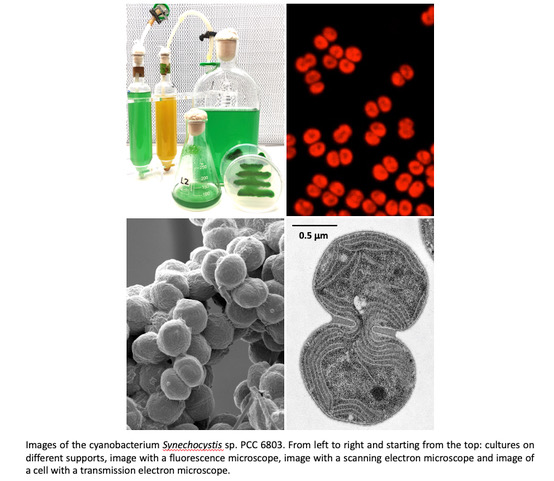
Francisco J. Florencio and M. Isabel Muro-PastorThe scientific interest of the group is focused on elucidating the adaptive strategies of cyanobacteria to changes in environmental conditions, especially those related to photosynthetic efficiency (light irradiance), nutrient availability (carbon and nitrogen) and changes in cellular redox state. For this purpose, we mainly use the unicellular cyanobacterium Synechocystis sp. PCC 6803, considered a model organism for photosynthesis and carbon metabolism studies, due to its metabolic plasticity and a deep knowledge of its genome.
We currently have several lines of research:
1. Regulation of central carbon metabolism.
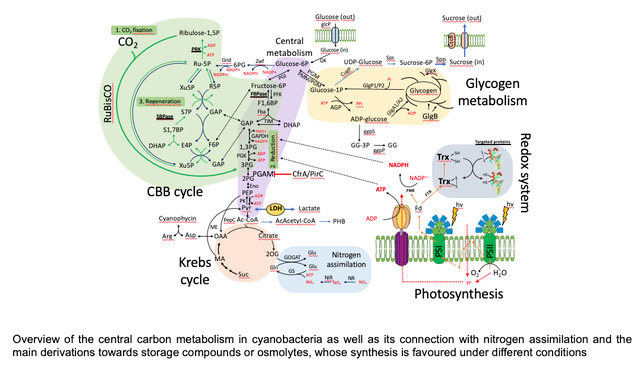
We analyze different mutants that alter cellular carbon flux, either by eliminating some metabolic pathway, as in the case of glycogen synthesis and degradation, or by modifying the capacity to regulate the flux itself, as in the case of the CfrA factor. The experimental approach includes metabolomic analyses, quantifying compounds of the central carbon metabolism (Calvin Cycle, Glycolysis, Krebs Cycle, and amino acid synthesis), enzymatic assays and expression of genes related to these processes. The aim is to build a model that adjusts to changes in illumination, CO2 levels and nitrogen availability in the medium, in order to identify the connectors of the different signals and how the integration between them occurs. As a corollary to all these studies, we are analyzing the biotechnological potential of the different mutants to produce carbon compounds, such as sucrose and lactate, with high efficiency by being able to control the flow of cellular carbon.
2. Dynamics of glycogen metabolism.
Using different mutants affected both in the synthesis and degradation of this polysaccharide, we analyze their influence on the cellular photosynthetic capacity and how carbon is redistributed according to the point of affectation of the process. We are interested in the effect that the production of glycogen granules of aberrant structure has on cell viability. We are also interested in identifying those proteins directly associated with the granule that may have a regulatory role in its dynamics. On the other hand, in mutants affected in glycogen synthesis, the metabolism of glucose, added externally, produces metabolic alterations that affect both the photosynthetic process and cell viability. Our aim is to determine the mechanisms that cause this interference.
3. Cellular redox state and photosynthetic metabolism.
In this line, a wide collection of mutants affected in the FTR-Trx (Ferredoxin thioredoxin reductase-Thioredoxin) redox system is studied. With respect to Trx, previous results obtained clearly indicate that the most abundant thioredoxin in cyanobacteria, TrxA (equivalent to plant thioredoxin m) is essential. Detailed analysis of a conditional TrxA mutant is allowing us to elucidate its multiple functions, most notably its interaction with the translational machinery. We are interested in the analysis of the mutants under different nutritional conditions (different CO2 concentrations and nitrogen sources). Through metabolic studies we intend to define their role in the control of the Calvin Cycle and in the distribution of sugars and amino acids depending on the level of TrxA. An objective within this line is the characterization of the mutants in the reductase (FTR), we already have conditional mutants, which present a pleiotropic character as occurs with the mutants in TrxA. A greater impact on cell viability is expected in these mutants since they should radically alter the global redox state.
4. Role of the exosome in adaptation to environmental stress.
Cyanobacteria are able to excrete metabolites, proteins and nucleic acids to the culture medium, as we have been able to analyze with several metabolic impairment mutants indicated in the previous lines. We intend to know in detail, how and when these compounds are excreted and if many of these compounds have a correlation with the environmental conditions in which the culture is located. In addition, previous studies in collaboration with other research groups suggest that Synechocystis is able to form extracellular vesicles that may have a relevant role in metal homeostasis. Knowing the conditions under which these vesicles are produced, and the nature of their contents may help us to understand the role they play in the adaptation to environmental conditions of these organisms.

| Name | Surname | Category | Phones | |
|---|---|---|---|---|
| Sandra | Díaz Troya | Doctor Professor Contract | 954139180 ext. 446068 |
|
| Francisco J. | Florencio Bellido | US Professor | 954489509 ext. 446009 |
|
| Joaquín | Giner Lamia | Professor Contract Doctor | 954489601 ext. 446003 |
|
| M. José | Huertas Romera | US Tenured Professor | 954489601 ext. 446003 |
|
| M. Isabel | Muro Pastor | CSIC Tenured Scientist | 954489598 ext. 446098 |
|
| Pablo | Ortega Martínez | Predoctoral Researcher | 954139180 ext. 446068 |
- Ortega-Martínez P, Nikkanen L, T. Wey L, Florencio FJ, Allahverdiyeva Y, Díaz-Troya S. Glycogen synthesis prevents metabolic imbalance and disruption of photosynthetic electron transport from photosystem II during transition to photomixotrophy in Synechocystis sp. PCC 6803. New Phytologist 2024. En prensa.
- Mallén-Ponce MJ, Florencio FJ, Huertas MJ. Thioredoxin A regulates protein synthesis to maintain carbon and nitrogen partitioning in cyanobacteria. Plant Physiol. 2024 Feb 22:kiae101. doi: 10.1093/plphys/kiae101.
- Domínguez-Lobo MT, Roldán M, Gutiérrez-Diánez AM, Florencio FJ, Muro-Pastor MI. Double blocking of carbon metabolism causes a large increase of Calvin-Benson cycle compounds in cyanobacteria. Plant Physiol. 2024 Feb 20:kiae083. doi: 10.1093/plphys/kiae083.
- Ortega-Martínez P, Roldán M, Díaz-Troya S, Florencio FJ. Stress response requires an efficient connection between glycogen and central carbon metabolism by phosphoglucomutases in cyanobacteria. J Exp Bot. 2023 Mar 13;74(5):1532-1550. doi: 10.1093/jxb/erac474. PMID: 36454663; PMCID: PMC10010611.
- Lima S, Matinha-Cardoso J, Giner-Lamia J, Couto N, Pacheco CC, Florencio FJ, Wright PC, Tamagnini P, Oliveira P. Extracellular vesicles as an alternative copper-secretion mechanism in bacteria. J Hazard Mater. 2022 Jun 5;431:128594. doi: 10.1016/j.jhazmat.2022.128594. Epub 2022 Feb 26. PMID: 35259694.
- Mallén-Ponce MJ, Huertas MJ, Sánchez-Riego AM, Florencio FJ. Depletion of m-type thioredoxin impairs photosynthesis, carbon fixation, and oxidative stress in cyanobacteria. Plant Physiol. 2021 Nov 3;187(3):1325-1340. doi: 10.1093/plphys/kiab321. PMID: 34618018; PMCID: PMC8566235.
- Bolay P, Rozbeh R, Muro-Pastor MI, Timm S, Hagemann M, Florencio FJ, Forchhammer K, Klähn S. The Novel PII-Interacting Protein PirA Controls Flux into the Cyanobacterial Ornithine-Ammonia Cycle. mBio. 2021 Mar 23;12(2):e00229-21. doi: 10.1128/mBio.00229-21. PMID: 33758091; PMCID: PMC8092223.
- García-Cañas R, Giner-Lamia J, Florencio FJ, López-Maury L. A protease-mediated mechanism regulates the cytochrome c6/plastocyanin switch in Synechocystis sp. PCC 6803. Proc Natl Acad Sci U S A. 2021 Feb 2;118(5):e2017898118. doi: 10.1073/pnas.2017898118. PMID: 33495331; PMCID: PMC7865156.
- Muro-Pastor MI, Cutillas-Farray Á, Pérez-Rodríguez L, Pérez-Saavedra J, Vega-de Armas A, Paredes A, Robles-Rengel R, Florencio FJ. CfrA, a Novel Carbon Flow Regulator, Adapts Carbon Metabolism to Nitrogen Deficiency in Cyanobacteria. Plant Physiol. 2020 Dec;184(4):1792-1810. doi: 10.1104/pp.20.00802. Epub 2020 Sep 8. PMID: 32900980; PMCID: PMC7723081.
- Robles-Rengel R, Florencio FJ, Muro-Pastor MI. Redox interference in nitrogen status via oxidative stress is mediated by 2-oxoglutarate in cyanobacteria. New Phytol. 2019 Oct;224(1):216-228. doi: 10.1111/nph.15979. Epub 2019 Jul 2. PMID: 31168850.


